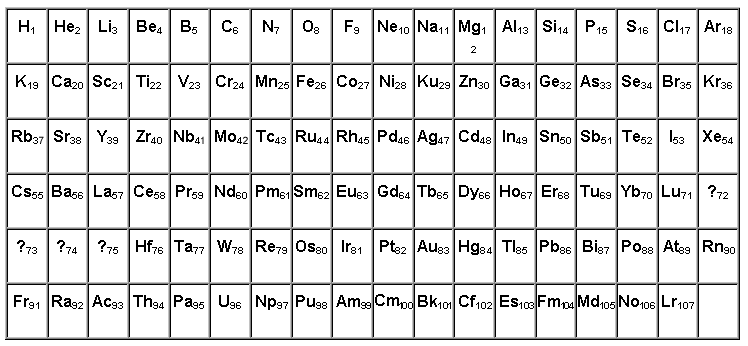
classification of all known chemical elements
Dear Sirs!
I give some explanations to my work. The atomic nucleus model was
developed in order to clarify the adjusted table of elements. Between
lutetium and hafnium, the difference in atomic masses does not reach
four units, while new elements with atomic numbers 72-75 are placed
there. How can nucleons be packed in a nucleus so that it is drip and
shell and with the necessary number of neutrons? Such a nucleus is
obtained if alpha particles are placed in the surface layer, and only
neutrons are inside the nucleus. In this case, in new chemical elements
with numbers 72-75 inside the nucleus, the neutron can be replaced by a
proton, and therefore the atomic mass of the elements between lutetium
and hafnium will vary slightly. The model was obtained by considering
the structure of the nuclei of atoms from heavy to light.
TABLE OF CHEMICAL ELEMENTS CONSTRUCTED ACCORDING TO THE CHARGES OF ATOMIC NUCLEI.
This article presents views on the classification of all known
chemical elements, those fundamental components that make up the Earth
and the entire Universe.
The innovation of this work lies in the fact that in the table of
elements, built according to Mendeleev's law and Van den Bruck's rule,
new chemical elements with atomic numbers 72-75 and 108-111 were
supposedly identified, and it was also shown that heavy elements,
starting from hafnium, the nuclei of atoms contain more protons than is
generally accepted.
A model of the atomic nucleus has been developed, which explains the
ratio of the number of protons to the number of neutrons. It is shown
why there are more neutrons in the nucleus than protons. All table cells
are filled.
If this table takes place, then I would like to name the groups of
elements with numbers 72-75 and 108-111, the islands of Filipenka H.R.
Probably James Chadwick made a mistake when measuring the charges of
atomic nuclei. More precisely, not an error in measurements, but in the
fact that he agreed with the periodic table and the result obtained for
platinum 77.6 was interpreted as a nucleus charge equal to 78, according
to the table. For copper, the result was 29.3 - more than true by 0.3,
for silver 46.3 is already less than true by 0.7, and for platinum it is
less than "true" by only 0.6. The decrease is due to the screening of
protons by each other during measurements. Therefore, for platinum with a
charge of 78, the result should have been less than that obtained, or
in other words, the platinum atom has a nuclear charge greater than 78
and equal to 82.
Let's build a model of the atomic nucleus. We know that there are
protons and neutrons in the nucleus. In each subsequent element, there
is more proton and several neutrons. Why? The volume grows faster than
the surface. With alpha radiation, helium nuclei of approximately the
same energies are emitted from the nucleus. Placing helium nuclei in the
surface layer of the atomic nucleus, we obtain with some accuracy that
the remaining neutrons are inside the nucleus. And the question is, can
and when is the proton inside the nucleus. According to Mendeleev's law
and Brook's rule, as well as the resulting model of the nucleus, a table
of elements is developed.
physical table of elements
Platinum is numbered 82 in this table. Protons begin to be located
inside the nucleus from 72 to 75 elements. Items not yet open. All cells
are filled in the table.
DI Mendeleev does not have a table, but a complex chemical structure.
Lanthanides and actinides, which should be arranged vertically
according to their chemical properties, are located horizontally under
the table in a "home" way. Brook's rule includes the periodic law and is
more general.
Please repeat the experiment of James Chadwick in measuring the
nuclear charge of the platinum atom. The charges of the copper and
silver nuclei are beyond doubt. But according to this table of elements,
built both according to Mendeleev's law and also according to van
Brook's rule, starting with hafnium, the charges of nuclei can be 4
units more than is accepted today with the same mass. To set the regimes
at nuclear power plants, it is probably important to know the true
charge of the uranium nucleus.
Dmitry Ivanovich intuitively felt that there should be a table of
elements, and not a complex structure, like his, but he probably did not
have enough knowledge of the structure of the atom and the nucleus of
an atom. Therefore, lanthanides and actinides are located horizontally.
The rule of Van den Bruck, an amateur nuclear physics, turned out to be
more general than the periodicity of Mendeleev and the calculations of
quantum mechanics. A table, by definition, must have all the cells
filled in according to a law or rule, and if you do not fill in any,
there must be an explanation of this by this law or rule.
Therefore, the cells of the physical table were filled as at http://physicaltable.blogspot.com
and unknown items numbered 72-75 and 108-111 appeared. Which demanded
an explanation. When reviewing the results of measuring the charges of
nuclei or atomic numbers by James Chadwick, I noticed that the charge of
the platinum nucleus is rather equal not to 78, but tends to 82, which
corresponds to the developed table. For almost 30 years I have been
raising the issue of repeating measurements of the charges of atomic
nuclei, since uranium probably has a higher charge than is accepted, but
it is used at nuclear power plants.
Lithium and beryllium, depending on temperature, change the crystal
lattice in much the same way as scandium and titanium. Which says about
the correctness of our table of chemical elements.
Bibliographic list: 1.G.G. Filipenko. "Suspicious" areas in periodic table, "Technology and Science", No4, Moscow, 1990.
2.G.G. Filipenko. A model of the atomic nucleus is proposed, "Engineer", No4, Moscow, 1991.
3. Papers of Independent Authors 2005 Issue 1, pp. 172-183.
4.Physical table of elements. http://physicaltable.blogspot.com
for those who did not understand ... there is an error in the
periodic table ... I do not know who made it by placing hafnium next to
lanthanum ... hafnium according to atomic weight and the following
elements should be in the table one line below ... then continuing with
lanthanides after lanthanum fill the cells and we get that in the
remaining 4 cells there are some not yet discovered new elements 72-75,
presumably with the chemical properties of lutetium. Since the electrons
corresponding to protons 72-75 will be distributed below the outer
electrons of lutetium. Quantum mechanics does not take into account the
structure and size of nuclei, so its conclusions are correct as long as
the nucleus can be taken as a point.
Everything about the same applies to elements 108-111.
who placed hafnium next to lanthanum and when ...
hafnium and the elements following them should be a line below according to changes in atomic weights in the table of elements ... in the table of Mendeleev's times, after lanthanum, there is cerium... and why?
I have the elements distributed in the long-period version of the table
according to the charges of the nuclei so as to fill all the cells of
the table
And how to distribute the isotopes of atoms according to the charge of the nuclei in the table.
isotopes
could be placed to the right and left of the element in a table that
would turn from a flat one into a volumetric one ... on the right, for
example, with a larger number of neutrons and on the left with a smaller
one ... and a certain body would be obtained from all elements and
their isotopes ... like -So maybe some properties could be found for a
system of all chemical elements ...
Henadzi Filipenka
1989. This table can be easily verified by repeating Sir Chadwick's experiments on measuring the charges of atomic nuclei. But on the planet there is such a science that no one needs anything as on the flying island of Laputa.
ReplyDeleteThe final version of the table of elements. https://dzen.ru/a/ZgZFzRw7tS5GDrM1
ReplyDelete